James Sturgis’ research group is interested in the dynamics and assembly of membrane proteins. We are interested in how membrane proteins fold and assemble in the membrane to form both stable structures, such as the photosynthetic apparatus, or transient dynamic structures.
To investigate the assembly of membrane proteins we use a combination of different theroetical and experimental techniques. In a series of numerical approaches we are using computer simulations and theoretical analysis to investigate the assembly process at various levels of detail (atomistic, coarse-grained and ultra-coarse grained). While at the experimental level we use spectroscopic, microscopic, molecular biology and synthetic biology methods to analyse the assembly process.
We have a series of biological systems that we investigate using these techniques: AqpZ, the Bacterial photosynthetic apparatus and mitochondrial anion channel, VDAC.
1 – AqpZ assembly
The membrane protein Aquaporin Z (AqpZ), from Escherichia coli forms a tetrameric water channel. However very little is known about the rules that govern membrane protein folding and assembly. We hope to be able to decode some of these rules by examining different steps in the folding of this protein.
Our research focusses on understanding the interactions between the protein and membrane lipids and in particular aspects of the protein surface and the lipid composition that modulate formation of tetrameric complexes and their subsequent assembly into 2D crystals. For this we are using various biochemical and biophysical techniques in the laboratory (fluorescence, FTIR spectroscopy, EM, lipidomics) or in collaboration with leading laboratories around the world (nmr, AFM and single molecule measurements).
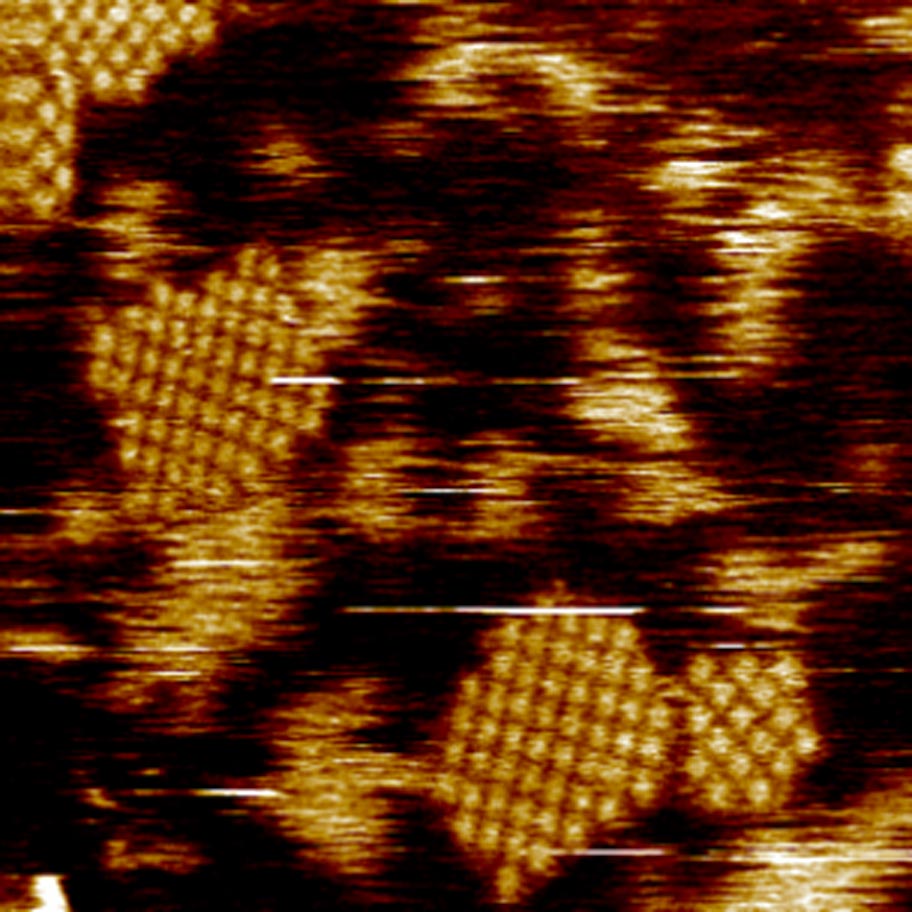
in a lipid membrane (Coll. S. Scheuring).
2 – Organization of the bacterial photosynthetic apparatus
A larger system we are studying is the bacterial photosynthetic system. This simple system forms specialized membranes composed essentially of 3 to 4 intergal membrane complexes. The different proteins are able to assemble spontaneously into specialise membranes within the bacteria, the morphology and composition of these domains are important for function, and we try to understand why certain proteins assemble into these domains and how their internal structure is determined.
Currently we are using multiple methods to modify the organization of membrane components, using molecular biology tools to modulate expression of the different components in living bacteria and constructing artificial photosynthetic systems bottom up. In both cases we study the organization and function of the system in order to elucidate the links between structure and function and the robustness of this simple integrated biological system.
3 – VDAC and Interactions
The Voltage-Dependent Anion Channel (VDAC) is the most abundant protein in the OMM. Its three isoforms form β-barrel porins governing the flux of ions and metabolites between the organelle and the cytosol. Although VDAC’s transport function has been thoroughly studied, its role in mitochondrial regulation, as well as the function of eachisoform, remains elusive.
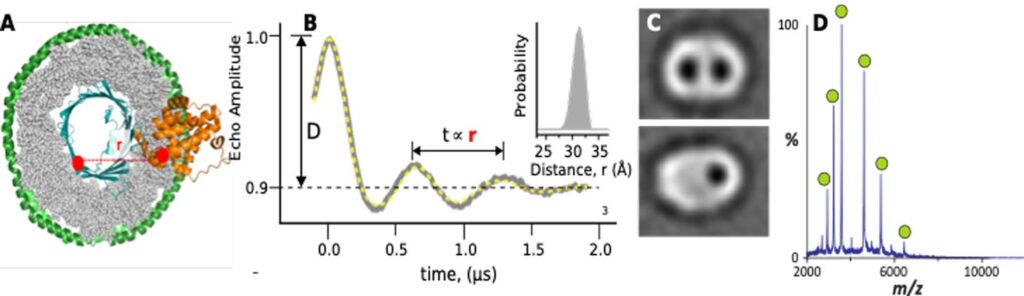
A. Hypothetical VDAC:Bax complex in nanodisc. Red dots are possible positions for DEER labeling. B. Typical DEER data. C. Negatively stained nanodiscs containing 1 or 2 VDAC channels. D. nMS spectrum of mVDAC1.
Numerous recent studies showed that interactions between pro-apoptotic Bax and Bak and VDAC are necessary to trigger cyt c release and the apoptosis cascade. Very limited data is available on the molecular mechanism of cyt c permeation. The characterization of the interaction between VDAC and Bax/Bak, and the complex(es) composition, formation or regulation are critical pieces missing to understand the molecular mechanism of this key event of apoptosis.
Our research focuses on identifying the exact composition, structure and regulation of VDAC and Bax/Bak pro-apoptotic complex(es). We use a combination of biochemical, biophysical (DEER, nMS, EM) and computational methods to investigate VDAC’s role in mitochondria-mediated apoptosis. This will expand our collective understanding of how these complexes assemble and trigger apoptosis, and establish hypothesis on the molecular mechanism behind cyt c release.
Members
Publications
VDAC interactions with lipids and their effect on isoform specificity
Jean-Pierre Duneau, Maria Queralt-Martin, Lucie Bergdoll
Biophysical Journal 122:373a (2023)10.1016/j.bpj.2022.11.2056
Nanodiscs as a novel approach to resolve inter-protein energy transfer within the photosynthetic membrane of purple bacteria
Olivia Fiebig, Dihao Wang, Dvir Harris, Hila Toporik, Yi Ji, Chern Chuang, Muath Nairat, Ashley Tong, John Ogren, Stephanie Hart, Jianshu Cao, James N. Sturgis, Yuval Mazor, Gabriela Schlau-Cohen
Biophysical Journal 122:232a (2023)10.1016/j.bpj.2022.11.1368
The single residue K12 governs highly sensitive voltage gating of the voltage-dependent anion channel
Van Ngo, Maria Queralt-Martin, Farha Khan, Lucie Bergdoll, Jeff Abramson, Sergey Bezrukov, Tatiana Rostovtseva, Sergei Noskov, David Hoogerheide
Biophysical Journal 122:92a (2023)10.1016/j.bpj.2022.11.697
Elucidating interprotein energy transfer dynamics within the antenna network from purple bacteria
Dihao Wang, Olivia Fiebig, Dvir Harris, Hila Toporik, Yi Ji, Chern Chuang, Muath Nairat, Ashley Tong, John Ogren, Stephanie Hart, Jianshu Cao, James Sturgis, Yuval Mazor, Gabriela Schlau-Cohen
Proceedings of the National Academy of Sciences of the United States of America 120:e2220477120 (2023)10.1073/pnas.2220477120
Membrane-mediated membrane protein interactions drive membrane protein organization
Yining Jiang, Batiste Thienpont, James N. Sturgis, Jeremy Dittman, Simon Scheuring
Biophysical Journal 121:433a (2022)10.1016/j.bpj.2021.11.608
Membrane-mediated protein interactions drive membrane protein organization
Yining Jiang, Batiste Thienpont, Vinay Sapuru, Richard Hite, Jeremy Dittman, James Sturgis, Simon Scheuring
Nature Communications 13:7373 (2022)10.1038/s41467-022-35202-8
Experimental evidence for long-distance electrodynamic intermolecular forces
Mathias Lechelon, Yoann Meriguet, Matteo Gori, Sandra Ruffenach, Ilaria Nardecchia, Elena Floriani, Dominique Coquillat, Frederic Teppe, Sébastien Mailfert, Didier Marguet, Pierre Ferrier, Luca Varani, James Sturgis, Jeremie Torres, Pettini Marco
Science Advances 8:eabl5855 (2022)10.1126/sciadv.abl5855
The Single Residue K12 Governs the Exceptional Voltage Sensitivity of Mitochondrial Voltage-Dependent Anion Channel Gating
Van A. Ngo, María Queralt-Martín, Farha Khan, Lucie Bergdoll, Jeff Abramson, Sergey m. Bezrukov, Tatiana K Rostovtseva, David P Hoogerheide, Sergei Yu Noskov
Journal of the American Chemical Society 144:14564-14577 (2022)10.1021/jacs.2c03316
Comparison of the Energy-Transfer Rates in Structural and Spectral Variants of the B800–850 Complex from Purple Bacteria
Ashley Tong, Olivia Fiebig, Muath Nairat, Dvir Harris, Marcel Giansily, Aurélia Chenu, James N. Sturgis, Gabriela Schlau-Cohen
Journal of Physical Chemistry B 124:1460-1469 (2020)10.1021/acs.jpcb.9b11899
High-speed atomic force microscopy highlights new molecular mechanism of daptomycin action
Francesca Zuttion, Adai Colom, Stefan Matile, Denes Farago, Frédérique Pompeo, Janos Kokavecz, Anne Galinier, James N. Sturgis, Ignacio Casuso
Nature Communications 11 (2020)10.1038/s41467-020-19710-z
The lipid environment of Escherichia coli Aquaporin Z
Victoria Schmidt, Marlon Sidore, Cherine Bechara, Jean-Pierre Duneau, James N. Sturgis
Biochimica et Biophysica Acta:Biomembranes 1861:431-440 (2019)10.1016/j.bbamem.2018.10.017
Out-of-Equilibrium Collective Oscillation as Phonon Condensation in a Model Protein
Ilaria Nardecchia, Jeremie Torres, Mathias Lechelon, Valeria Giliberti, Michele Ortolani, Philippe Nouvel, Matteo Gori, Yoann Meriguet, Irene Donato, Jordane Preto, Luca Varani, James N. Sturgis, Marco Pettini
Physical Review X 8:031061 (2018)10.1103/physrevx.8.031061
Modifying Styrene-maleic Acid Co-polymer for Studying Lipid Nanodiscs by Direct Fluorescent Labeling
Victoria Schmidt, James N. Sturgis
Bio-protocol 8 (2018)10.21769/BioProtoc.2969
Modifying styrene-maleic acid co-polymer for studying lipid nanodiscs
Victoria Schmidt, James N. Sturgis
Biochimica et Biophysica Acta:Biomembranes 1860:777-783 (2018)10.1016/j.bbamem.2017.12.012
Making Monomeric Aquaporin Z by Disrupting the Hydrophobic Tetramer Interface
Victoria Schmidt, James N. Sturgis
ACS Omega 2:3017-3027 (2017)10.1021/acsomega.7b00261
Lipid perturbation by membrane proteins and the lipophobic effect
James N. Sturgis, Jean-Pierre Duneau, Jonathan Khao
Biochimica et Biophysica Acta:Biomembranes 1859:126-134 (2017)10.1016/j.bbamem.2016.10.014
Making dimers of oligomeric membrane proteins using copper-free click chemistry
James N. Sturgis, Wang Dong
F1000Research 5 (2016)10.12688/f1000research.8676.1
Lipid Environment of Aquaporin Z
Schmidt Victoria, Sidore Marlon, Carrière Fredéric, Duneau Jean-Pierre, James N. Sturgis
Biophysical Journal 110:252a-253a (2016)10.1016/j.bpj.2015.11.1389
The E.coli Aquaporin Z interface : a puzzle?
Victoria Schmidt, Pierre Hubert, James N. Sturgis
Eur. Biophys. J. 44:S217 (2015)
Transmembrane Recognition of the Semaphorin Co-Receptors Neuropilin 1 and Plexin A1 : Coarse-Grained Simulations
Samia Aci-Sèche, Paul Sawma, Pierre Hubert, James N. Sturgis, Dominique Bagnard, Laurent Jacob, Monique Genest, Norbert Garnier
PLoS ONE 9:e97779 (2014)10.1371/journal.pone.0097779
Ultrafast excited state processes in Roseobacter denitrificans antennae: comparison of isolated complexes and native membranes.
Marco Ferretti, Katia Duquesne, James N. Sturgis, Rienk Van Grondelle
Physical Chemistry Chemical Physics 16:26059-66 (2014)10.1039/c4cp02986k
Evidence for new homotypic and heterotypic interactions between transmembrane helices of proteins involved in receptor tyrosine kinase and neuropilin signaling.
Paul Sawma, Lise Roth, Cécile Blanchard, Dominique Bagnard, Gérard Crémel, Emmanuelle Bouveret, Jean-Pierre Duneau, James N. Sturgis, Pierre Hubert
Journal of Molecular Biology 426:4099-111 (2014)10.1016/j.jmb.2014.10.007
The architecture of Rhodobacter sphaeroides chromatophores
Simon Scheuring, Reinat Nevo, Lu-Ning Liu, Stéphanie Mangenot, Dana Charuvi, Thomas Boudier, Valerie Prima, Pierre Hubert, James N. Sturgis, Ziv Reich
Biochimica biophysica acta (BBA) - Bioenergetics 1837:1263-1270 (2014)10.1016/j.bbabio.2014.03.011
Destabilizing Aquaporin Z Assembly: Effects on Structure, Function and Dynamics
Victoria Schmidt, Pierre Hubert, Valerie Prima, James N. Sturgis
Biophysical Journal 108:499a-500a (2014)
Molecular mechanisms of Tau binding to microtubules and its role in microtubule dynamics in live cells.
Gilles Breuzard, Pierre Hubert, Roqiya Nouar, Tiphany De Bessa, François Devred, Pascale Barbier, James N. Sturgis, Vincent Peyrot
Journal of Cell Science 126:2810-9 (2013)10.1242/jcs.120832
Lateral organization of biological membranes: role of long-range interactions.
Jean-Pierre Duneau, James N. Sturgis
Eur. Biophys. J. 42:843-50 (2013)10.1007/s00249-013-0933-x
Structural properties of the tubular appendage spinae from marine bacterium Roseobacter sp. strain YSCB.
A Bernadac, L-F Wu, C-L Santini, C Vidaud, James N. Sturgis, N Menguy, P Bergam, C Nicoletti, T Xiao
Scientific Reports 2:950 (2012)10.1038/srep00950
Characterization of the motion of membrane proteins using high-speed atomic force microscopy
Ignacio Casuso, Jonathan Khao, Mohamed Chami, Perrine Paul-Gilloteaux, Mohamed Husain, Jean-Pierre Duneau, Henning Stahlberg, James N. Sturgis, Simon Scheuring
Nature Nanotechnology 525-529 (2012)10.1038/nnano.2012.109
Complete Lateral and Angular Diffusion and Protein-Protein Interaction Description of a Membrane Protein
Ignacio Casuso, Jean-Pierre Duneau, Mohamed Chami, Perrine Paul-Gilloteaux, Mohamed Husain, Jonathan Khao, Henning Stahlberg, James N. Sturgis, Simon Scheuring
Biophysical Journal 102:413a-414a (2012)10.1016/j.bpj.2011.11.2261
Draft genome sequence of the purple photosynthetic bacterium Phaeospirillum molischianum DSM120, a particularly versatile bacterium.
K Duquesne, Valérie Prima, B Ji, Z Rouy, C Médigue, E Talla, James N. Sturgis
Journal of Bacteriology 194:3559-60 (2012)10.1128/JB.00605-12
Shotgun genome sequence of the large purple photosynthetic bacterium Rhodospirillum photometricum DSM122.
K Duquesne, James N. Sturgis
Journal of Bacteriology 194:2380 (2012)10.1128/JB.00168-12
Molecular origins and consequences of High-800 LH2 in Roseobacter denitrificans.
Katia Duquesne, Cecile Blanchard, James N. Sturgis
Biochemistry 50:6723-9 (2011)10.1021/bi200538j
Structure of a protein-detergent complex: the balance between detergent cohesion and binding.
Jonathan Khao, Jaime Arce-Lopera, James N. Sturgis, Jean-Pierre Duneau
Eur. Biophys. J. 40:1143-55 (2011)10.1007/s00249-011-0745-9
Native architecture of the photosynthetic membrane from Rhodobacter veldkampii.
Lu-Ning Liu, James N. Sturgis, Simon Scheuring
Journal of Structural Biology 173:138-45 (2011)10.1016/j.jsb.2010.08.010
Antagonistic regulation of dgkA and plsB genes of phospholipid synthesis by multiple stress responses in Escherichia coli.
Astrid Wahl, Laetitia My, Romain R. Dumoulin, James N. Sturgis, Emmanuelle Bouveret
Mol. Microbiol. 80:1260-75 (2011)10.1111/j.1365-2958.2011.07641.x
The effects of protein crowding in bacterial photosynthetic membranes on the flow of quinone redox species between the photochemical reaction center and the ubiquinol-cytochrome c2 oxidoreductase.
Kamil Woronowicz, Daniel Sha, Raoul N Frese, James N. Sturgis, Vikas Nanda, Robert A Niederman
Metallomics 3:765-74 (2011)10.1039/c1mt00034a
High-resolution architecture of the outer membrane of the Gram-negative bacteria Roseobacter denitrificans.
Szymon Jarosławski, Katia Duquesne, James N. Sturgis, Simon Scheuring
Molecular Microbiology 74:1211-1222 (2009)10.1111/j.1365-2958.2009.06926.x
Rows of ATP Synthase Dimers in Native Mitochondrial Inner Membranes
Nikolay Buzhynskyy, Pierre Sens, Valérie Prima, James N. Sturgis, Simon Scheuring
Biophysical Journal 93:2870-2876 (2007)10.1529/biophysj.107.109728
Confined diffusion in tubular structures analyzed by fluorescence correlation spectroscopy on a mirror
E. Etienne, P.-F. Lenne, James N. Sturgis, H. Rigneault
Applied optics 45:4497-4507 (2006)10.1364/AO.45.004497
The photosynthetic apparatus of Rhodopseudomonas palustris: Structures and organization.
Simon Scheuring, Rui Pedro Goncalves, Valérie Prima, James N. Sturgis
Journal of Molecular Biology in press:in press (2006)
The TolQ-TolR proteins energize TolA and share homologies with the flagellar motor proteins MotA-MotB
E. Cascales, Roland Lloubès, James N. Sturgis
Molecular Microbiology 42:795-807 (2001)10.1046/j.1365-2958.2001.02673.x
Proton motive force drives the interaction of the inner membrane TolA and outer membrane Pal proteins in Escherichia coli
E. Cascales, Marthe Gavioli, James N. Sturgis, Roland Lloubes
Molecular Microbiology 38:904-915 (2000)10.1046/j.1365-2958.2000.02190.x
1H-13C nuclear magnetic resonance assignment and structural characterization of HIV-1 Tat protein.
Jean-Marie Peloponese, C. Gregoire, S Opi, E Esquieu, James N. Sturgis, E Lebrun, E. Meurs, C Collette, D Olive, M Aubertin, M Witvrow, C Pannecouque, E de Clercq, Cedric Bailly, J. Lebreton, E. Loret
Comptes rendus de l’Académie des sciences. Série III, Sciences de la vie 323:883-94 (2000)10.1016/S0764-4469(00)01228-2
Collaborations
- National
- Olivia du Roure, ESPCI ParisTech
- Julien Heuvingh, ESPCI ParisTech
- Clément Campillo, Université d’Evry
- Guido Pintacuda, CNRS ISA, Lyon
- Marco Pettini, CNRS CPT, Marseille
- Ignacio Casuso, INSERM, Marseille
- International
- David Kovar University of Chicago, USA
- Robert Robinson IMCB, Singapore
- Simon Scheuring, Corning Medical School, USA
- Janice Robertson, Washington University, USA
- Gabriela Schlau-Cohen, MIT, USA
- Alexandra Olaya-Castro, UCL, UK
- Luca Sapienza, Southampton University, UK
Funding